Influenza Series
Influenza (hereinafter referred to as flu) is an acute respiratory infectious disease caused by the influenza virus, which belongs to the family Orthomyxoviridae and is a single-stranded negative-sense RNA virus. Based on the characteristics of the virus's nucleoprotein (NP) and matrix protein (M), influenza viruses are classified into four types: A, B, C, and D, with type A being the most pathogenic to humans, exhibiting the strongest virulence and a high mutation rate, followed by type B influenza virus. The influenza virus is primarily transmitted through droplets in the air, contact between susceptible individuals and infected individuals, or contact with contaminated objects. Type A influenza virus (Influenza A, hereinafter referred to as FLU A) is divided into 18 HA subtypes and 11 NA subtypes based on the differences in the surface proteins hemagglutinin (HA) and neuraminidase (NA). The H1N1 and H3N2 subtypes of type A are the main subtypes causing seasonal flu. Type B influenza virus (Influenza B, hereinafter referred to as FLU B) can be divided into two lineages based on hemagglutinin HA: the B/Victoria lineage (named after the B/Victoria/2/1987 strain) and the B/Yamagata lineage (named after the B/Yamagata/16/1988 strain). Currently, the circulating strain in the population is the B/Victoria lineage. Nucleoprotein NP is the main structural protein of the influenza virus, highly conserved, with specificity for populations and types, and is an important basis for the classification of influenza viruses. It is often used in the immunodiagnosis of influenza viruses and in the research and development of new influenza virus vaccines. Xiamen Tongrenxin Biotechnology Co., Ltd (hereinafter referred to as "Tongrenxin") has rich experience in developing antibodies against type A and B influenza pathogens and has launched high-affinity antibody pairs targeting the NP protein of type A and B influenza viruses. Additionally, they have introduced rapid detection kits (colloidal gold) for type A and B influenza virus antigens for corporate clients to choose from.
Product Performance
(1) Sensitivity Assessment
Using materials prepared with Renxin ingredients to detect inactivated influenza viruses and national positive reference samples, the results are as follows:
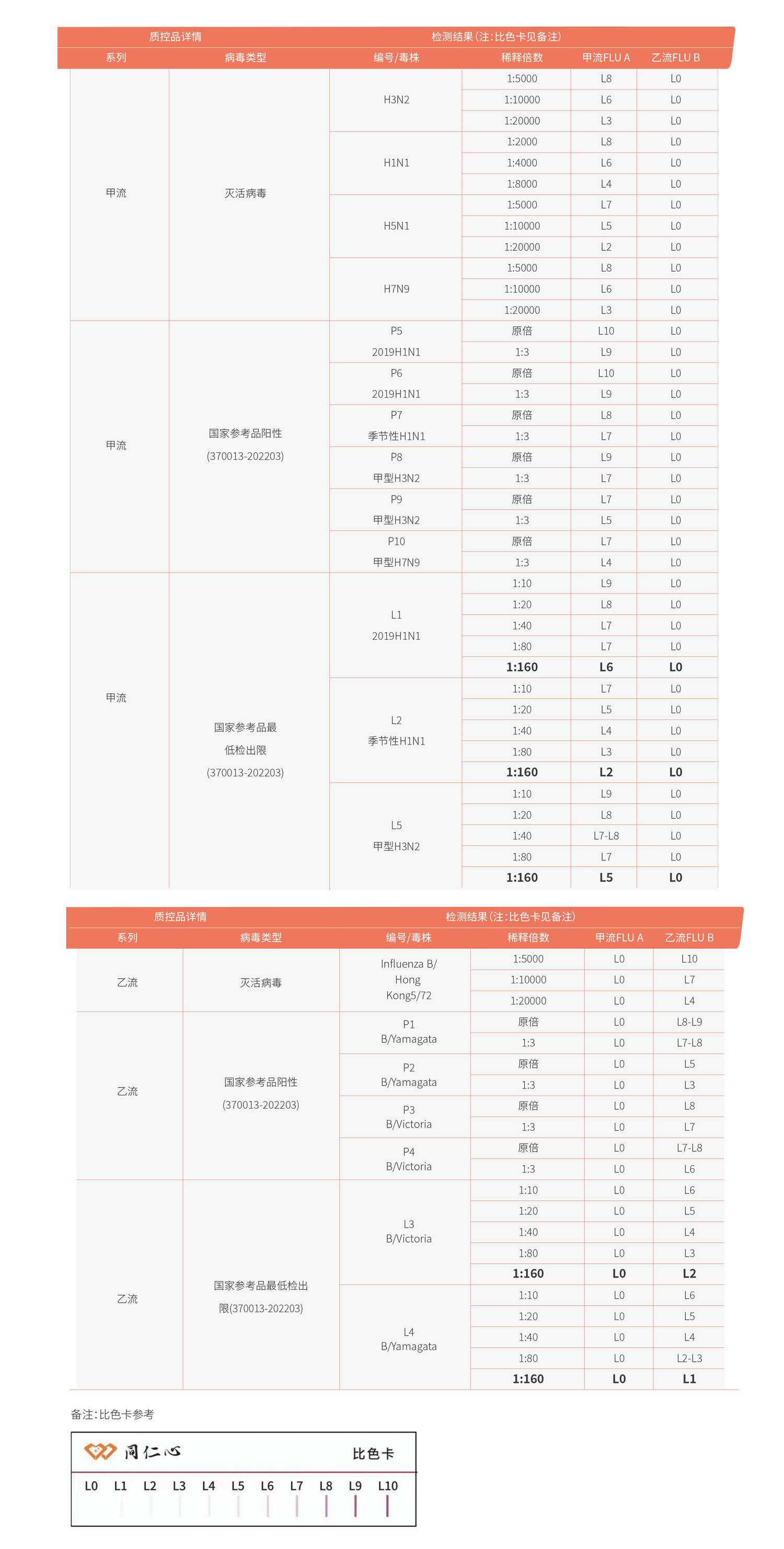
[1] Vemula S V, J Zhao, J Liu, X Wang, et al. Current Approaches for Diagnosis of Influenza Virus Infections in Humans [J]. Viruses, 2016, 8(4): 96.
[2] Hay A J, Gregory V, Douglas A R, et al. The evolution of human influenza viruses [J]. Philos Trans R Soc Lond B Biol Sci. 2001, 356(1416): 1861-70.
[3] Maja S A, Katarina P, Veronika U A, et al. A comparison of the demographic and clinical characteristics of laboratory-confirmed influenza B Yamagata and Victoria lineage infection [J]. Journal of Clinical Virology the Official Publication of the Pan American Society for Clinical Virology, 2014, 61(1): 156-160.
[4] Cianci C, Gerritz S W, Deminie C, et al. Influenza nucleoprotein: promising target for antiviral chemotherapy [J]. Antivir Chem Chemother. 2012, 23(3): 77-91.
[5] Loregian A, Mercorelli B, Nannetti G, et al. Antiviral strategies against influenza virus: towards new therapeutic approaches [J]. Cell Mol Life Sci. 2014. 71(19): 3659-83.
*NIBSC (National Institute for Biological Standards and Control) is the UK National Biological Products Testing Laboratory and an international standard supply center laboratory for WHO. Its core work is to prepare, preserve, and distribute WHO standards for testing the quality of biological products globally.
*National and global influenza surveillance data can be found at the China National Influenza Center (https://ivdc.chinacdc.cn/cnic/).



